Notes on Hydrogen
A recent conversation about electrolysis motivated me to get a basic understanding of sources and uses of H2 as relevant to climate and energy.
These are quick, rough notes. May have significant omissions or misunderstandings. Please tweet or email me if so! Not meant as an authoritiative reference, but hopefully helpful for those looking to get a basic understanding.
Thanks very much to the folks at Charm Industrial for explaining this to me on a Sunday afternoon, and answering my very uninformed questions!
For additional accessible reading, check out this CarbonBrief article.
I've heard climate and energy people talk about hydrogen for a while now, without really understanding why it's relevant. A recent conversation about electrolysis motivated me to get a basic understanding of sources and uses of H2 as relevant to climate and energy.
What is hydrogen?
The first element of the periodic table. Glows a pretty purple if you pass electricity through it. Makes the sun go.
At standard temperature and pressure, it's a colorless odorless gas as H2. All your basic element details on Wikipedia.
How do we use hydrogen?
Use it chemically
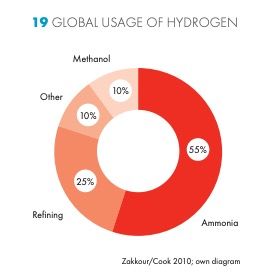
As an ingredient in common industrial reactions, H2 can be used chemically to refine crude oil, produce ammonia, plastics, glass etc.
The "produce ammonia" case is a world-changingly big deal. This is via the Haber-Bosch process, which takes H2 (along with nitrogen) and makes ammonia (NH3), an essential ingredient in fertilizers responsible for dramatically increasing the carrying capacity of the planet.
H2 is also used for Hydrotreating and Hydrocracking, important components of oil refining.
Use it in fuel cells
In fuel cells, hydrogen is used to produce electricity. This is usually for use in cars and other transport applications. Fuel cells aren't anywhere close to the scale of e.g. batteries, but car companies like Toyota and Honda are actively building fuel cell cars for the mass market. Fuel cell vehicles have been a niche market for decades.
My first thought was: isn't driving around with a tank of explosive H2 a bad idea? Turns out that it's less explode-y than gasoline.
Compared to battery-electric cars, hydrogen fuel-cell electric cars are much faster to refuel (you're just pumping H2 into a tank instead of charging a battery) and have longer range. On the other had, H2 pumps at gas stations aren't nearly as widespread as charging stations, and I haven't seen a fuel cell car close to as cool as a Tesla.
For climate, there's not a clear winner on fuel cell electric vs battery electric cars – the carbon intensity of grid electricity varies wildly for battery cars, as does the carbon intensity of hydrogen production. But, in theory, both technologies could be zero-emission. Here's a study that assumes some stuff about price curves and says battery electric cars are better for emissions starting in 2025. Here's an article summarizing that study. Note that "research was supported by BMW", I'm not sure which direction that could bias it.
Oil companies are, as you might expect, exploring diversifying into hydrogen: see this (genuinely informative) overview study from Shell. (The Shell station on the edge of South Park in SF recently installed a hydrogen pump...).
This is an area I'd like to learn more about: what's the cost and innovation curve look like for fuel cells? How much better have they gotten over the last decade? How much better could they theoretically get? Do we need brand new materials science, or just simple-ish manufacturing scaling?
Burn it
While you can burn H2 as a source of heat, for electricity generation etc, it's a low value use case. Among other reasons, this is because it's lower energy density than natural gas/oil/coal etc, along with being difficult to transport. There are many better heat sources.
One other thing: unlike e.g. oil or natural gas (CH4), it's generally not economical to transport hydrogen (not dense enough to make sense to transport via truck, too corrosive and flammable to transport via pipeline, etc). So, for most use cases, hydrogen is produced at point-of-use.
Where does Hydrogen come from?
While it's a very abundant element in the universe overall, the hydrogen molecules we use come from these two processes:
Steam (methane) reformation (~99% of the market)
This is a term I'd heard thrown around a lot, but wasn't sure what it meant or where it fit into various energy processes.
Basically – you heat up natural gas along with water, pipe it through some complicated machines, and produce standalone Hydrogen (alongside carbon monoxide). Here's an overview.
In brief, the reaction looks like this:
In practice, the reaction looks like this:

Here's an (oversimplified) break out of the steps:
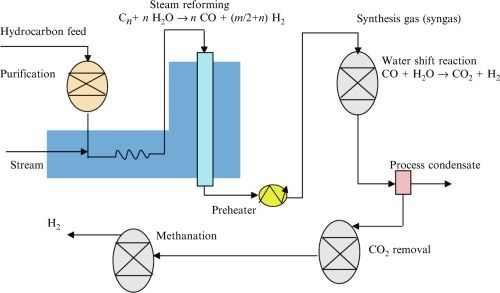
The steam reformation process produces syngas (CO + CO2 + H2 + H2O), which is worth understanding a bit about. Syngas is used to synthesize a number of important products.
We can perform "shift reactions" on the syngas, and make everything from Deisel to Methanol to Hydrogen.
In the figure above we can think of "hydrocarbon feed" as methane (CH4). After the yellow zig-zag, we transition through syngas. With a "shift reaction" (in Hydrogen's case, water shift), we can yield H2.
Electrolysis (~1% of the market)
Basically: use electricity to break the hydrogen bond in water (H2O) to yield gaseous H2.
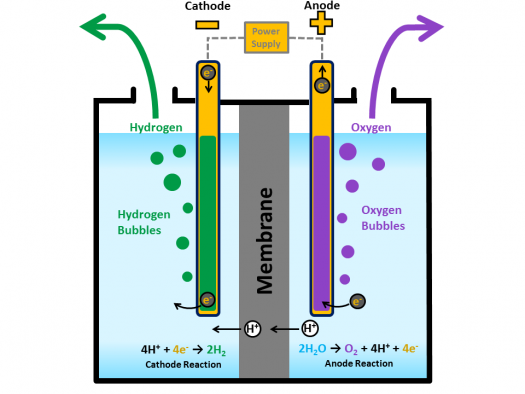
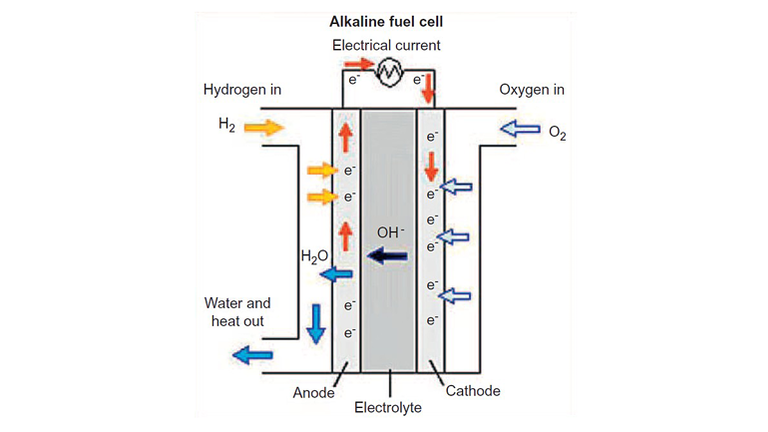
There are many different kinds of electrolyzers with various costs and efficencies. The above figure shows the common PEM (polymer electrolyte membrane) cell. Here's a good resource to learn more about the different types, and fuel cells overall.
Originally, I assumed lots more than 1% of our hydrogen came from electrolysis. It turns out electrolysis is currently prohibitively expensive (high capex due to the electrolyzers themselves being expensive machines, high opex due to the often-low efficency -> big electric bill to run them). Cheaper electrolyzers would be great!
Market Size
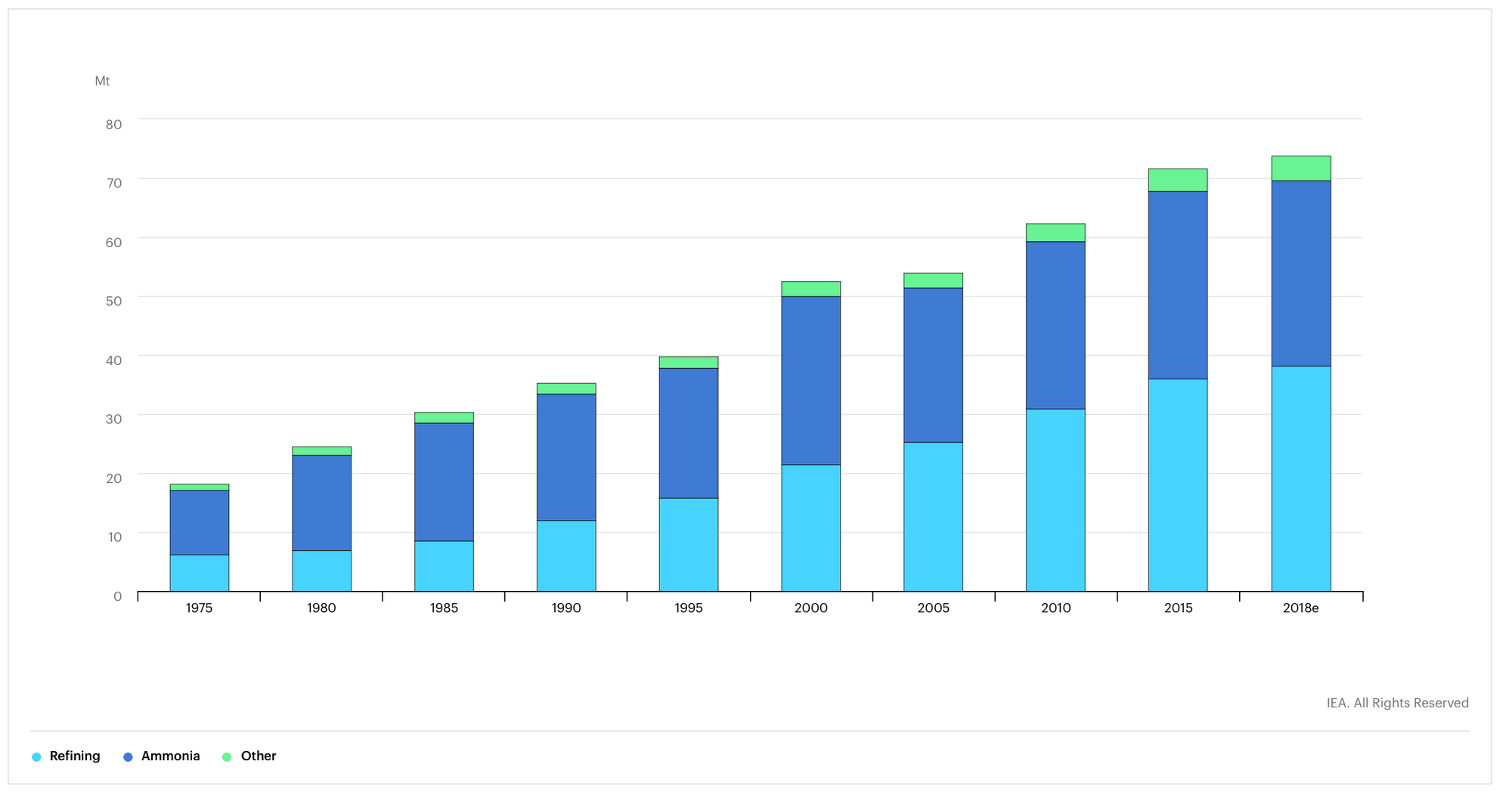
The world produces between 65 and 100 megatons of H2 a year, of which the U.S. produces about 10 megatons. (Apparently "on-purpose" [...hydrogen production] is as a technical term, which I think is cute). Total demand for hydrogen is a bit over 70 megatons a year (source).
Prices vary, but somewhere in the range of $1500/ton is commonly cited for industrial H2. Here's a good summary of a 2019 paper outlining the latest cost curve, which has some great additional links and explanations as well.
Prices for H2 at the pump for consumer cars is are about 10x that, due to a combination of costs of compression and transport (hence why, for most industrial uses, you'd want to produce the H2 on-site). Pump units are in kilograms:
Hydrogen fuel prices range from $12.85 to more than $16 per kilogram (kg), but the most common price is $13.99 per kg (equivalent on a price per energy basis to $5.60 per gallon of gasoline), which translates to an operating cost of $0.21 per mile.
(Source of the above on page 7, it's from 2015, I'm not sure how much has changed since then but this seems ballpark right.)
Across both, the H2 market is on the order of $150 billion a year. That's meaningful!
